On March 21, a notice was posted on the Fort Dix website, addressed to soldiers serving at that US Army post near the city of Trenton, in Mercer County, New Jersey.
The Joint Base McGuire-Dix-Lakehurst commander has implemented HPCON CHARLIE for JB MDL to protect the health and safety of its members, families and surrounding community. JB MDL remains essential personnel only (if you do not live on base or have an official and necessary purpose to enter the installation, please stay home for the safety of you and others). This is a substantial health alert in response to continued and increased public health risks in the local community and surrounding major metropolitan areas…
‘JB MDL’ translates as ‘JOINT BASE MCGUIRE-DIX-LAKEHURST’… and ‘HPCON CHARLIE’ translates as ‘HEALTH PROTECTION CONDITION C’:
As of May 3, Mercer County, New Jersey, was reporting 4,743 cases of COVID-19, with the number doubling about every two weeks. In terms of the rate of coronavirus spread, Mercer County appears to be about average for East Coast. Reportedly, there have been 280 COVID deaths in the county.
I was researching Fort Dix, because that particular Army base played a minor role in the events of 1976 — the ultimately embarrassing ‘National Influenza Immunization Program’. Those events might provide some perspective on the current COVID-19 situation.
From the Washington Post, April 29, 2020:
The Trump administration is racing to develop a coronavirus vaccine that could be fielded nationwide by January, U.S. officials said Thursday, as national stay-at-home guidance expired. The January timeline represents a fast pace for vaccine development but still means there would be no fail-safe protection from the novel coronavirus until long after most Americans are likely to have returned to work or school and until after the November presidential election.
Anthony S. Fauci, the United States’ top infectious-disease specialist, said the goal is production of hundreds of millions of doses by January, an effort dubbed “Operation Warp Speed.”
“We want to go quickly, but we want to make sure it’s safe and it’s effective,” he said on NBC’s ‘Today’ show. “I think that is doable if things fall in the right place.”
Fauci, the director of the National Institute of Allergy and Infectious Diseases, said manufacturers of the best potential vaccine candidates would ramp up production “at risk,” meaning before they are proven to work, to speed up the process…
We’ve been here before, as it turns out. The outcome was not so good, except for the pharmaceutical industry.
In January 1976, several Fort Dix soldiers complained of a respiratory illness diagnosed as influenza. The following month, Private David Lewis, who had been showing flu symptoms, participated in a five-mile forced march, collapsed, and died. The New Jersey Department of Health then tested samples from the Fort Dix soldiers, and while the majority of samples were of the more common A Victoria flu strain, two were not. The atypical samples were sent to the Centers for Disease Control (CDC) in Atlanta, Georgia, which classified them as ‘Swine influenza A’.
Apparently, pigs — all around the globe — commonly carry a range of influenza viruses, including subtypes of influenza A known as H1N1, H1N2, H2N1, H3N1, H3N2, and H2N3. Occasionally, pig influenza virus gets transmitted to humans.
The scientists at the CDC got rather excited in February 1976, because they concluded that the atypical virus found in two Fort Dix soldiers seemed similar to the virus that had allegedly caused the 1918 influenza pandemic — a global disaster which killed perhaps 100 million people worldwide. The CDC informed the World Health Organization and the state of New Jersey of the potential danger, and on February 13, CDC Director David Sencer completed a memo calling for mass immunization for the swine flu.
President Gerald Ford was informed of the memo on March 15, and he met with a “blue ribbon” panel which included Jonas Salk and Albert Sabin, the then-famous inventors of two popular polio vaccines. The President followed that meeting with a televised announcement in support of a mass federally-sponsored immunization program. During a subsequent hearing before a US Senate Appropriations Subcommittee, a drug company spokesman requested government indemnity for the vaccine manufacturers. Pharmaceutical companies Merck & Co, Merrell, Wyeth, and Parke-Davis announced their refusal to sell doses to the government unless they were guaranteed a profit.
The House of Representatives approved $135 million for the swine flu immunization program on April 5. A little more than two weeks later, the Ford Administration submitted a proposal to Congress offering indemnity to vaccine manufacturers, and production of an H1N1 vaccine commenced.
On July 1, CDC Assistant Director for Programs, Bruce Dull, stated at a public health conference that there were no parallels between the 1918 flu pandemic and the current situation. Later that month, J. Anthony Morris, a Food and Drug Administration researcher, was dismissed for insubordination and went public with his findings that cast doubt on the safety of the vaccine. Three days later, several manufacturers announced they had ceased production of the vaccine. In the latter part of the month, investigations into alleged swine flu outbreaks in other parts of the world found no cases of the Fort Dix strain.
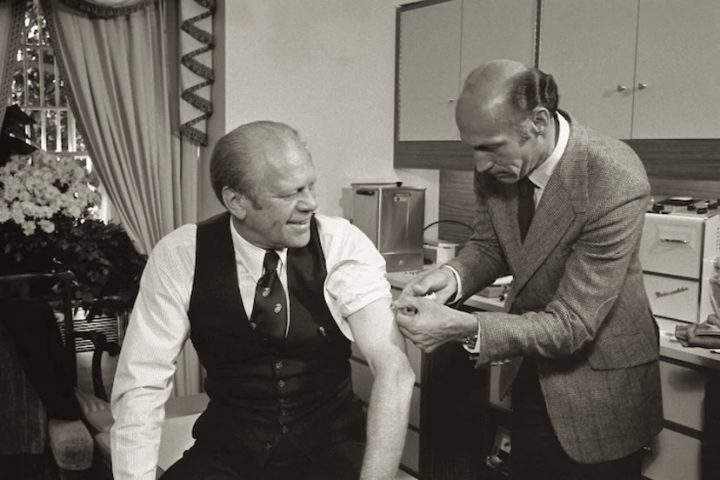
On August 6, President Ford held a press conference urging Congress to take action on the indemnification legislation. Four days later, both Houses of Congress passed the legislation, allowing US drug companies to mass produce 50 million doses of an essentially untested vaccine — with no risk of liability. Merck made the first shipment of vaccines to state health departments on September 22 and the first swine flu inoculations were given at the Indiana State Fair. Subsequently, cases of Guillain-Barré Syndrome (GBS) in vaccinated patients were reported in several states, including Minnesota, Maryland, and Alabama. More cases of Guillain-Barré were reported in early December and the investigation into GBS cases spread to eleven states.
On December 16, a one-month suspension of the vaccination program was announced by CDC Director Sencer. On February 4, Sencer was informed that he would be replaced as the head of the CDC.
The immunization program was not reinstated. But 45 million Americans had already allowed themselves to be vaccinated against a flu strain with a very low reproductive rate and mild symptoms.
And the US pharmaceutical industry learned how to make a huge profit selling an ineffective, federally-sponsored vaccine, with zero legal liability.
